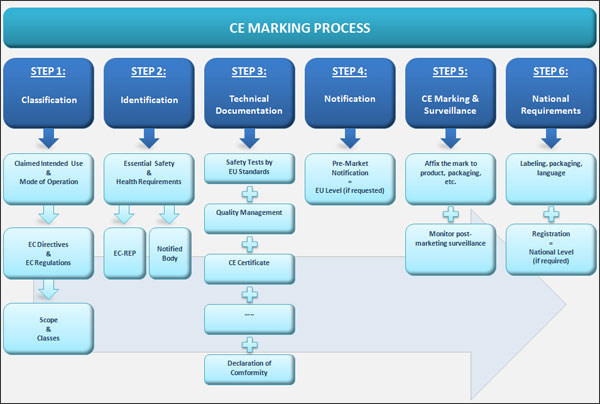
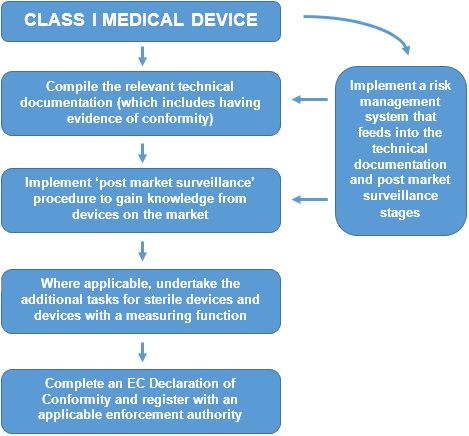
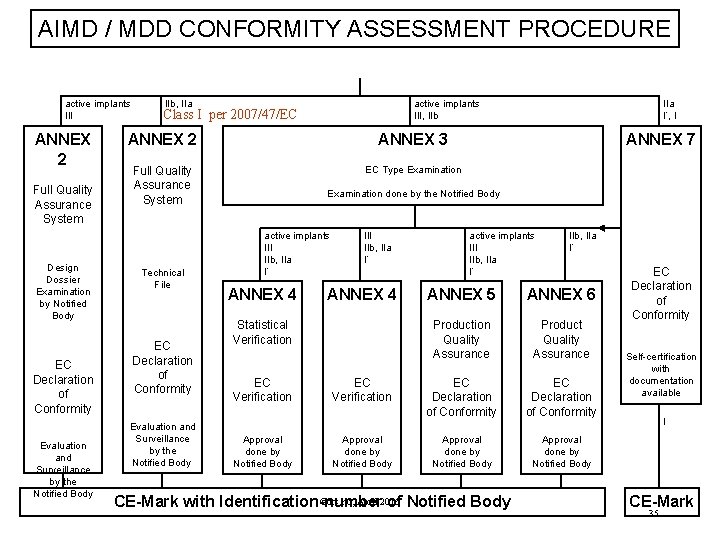
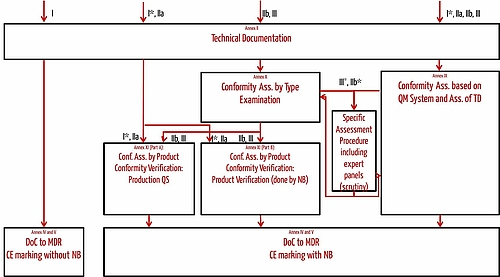
Classification Of Medical Devices And Their Routes To CE Marking – Clever Compliance Support - Compliance system and CE marking information

DEVICE REGULATIONS - The New Medical Device Regulation & the Applicability of Article 117 to Medicinal Products

Classification Of Medical Devices And Their Routes To CE Marking – Clever Compliance Support - Compliance system and CE marking information